Adorable! Why Does Entropy Always Increase
What does a change in entropy mean and why should we be interested in it. Thus entropy measurement is a way of distinguishing the past from the future.

4 Laws Of Thermodynamics Explained Thermodynamics Science Anchor Charts Physics Formulas
We see evidence that the universe tends toward highest entropy many places in our lives.

Why does entropy always increase. More specifically the second law of thermodynamics states that as one goes forward in time the net entropy degree of disorder of any isolated or closed system will always increase or at least stay. X ˆ 0 with prob p 1 with prob 1p 2 then the entropy of X is given by HX plogp1plog1p Hp 3 Note that the entropy does not depend on the values that the random variable takes 0 and 1. 22 Entropy increase de nes arrow of time This should be very puzzling for everybody because all microscopic theories of nature eg.
Ice melting salt or sugar dissolving making popcorn and boiling water for tea are. Putting The Pieces Together. As Chestermiller has just pointed out youve stated the expression for entropy change of an ideal gas at constant T.
When changes occur in a chemical reaction they are nearly always accompanied by changes in the surroundings. Why is the PH of a base bigger than 7SecondAt the equivalence point of a titration between acetic acid weak acid and sodium hydroxide strong base. So why does time always move forward.
The measure of such a disorganized motion of molecules is called the entropy denoted by S. A campfire is an example of entropy. The only way to make things orderly again is to add energy.
The term and the concept are used in diverse fields from classical thermodynamics where it was first recognized to the microscopic description of nature in statistical physics and to the principles of information theory. Entropy is a scientific concept as well as a measurable physical property that is most commonly associated with a state of disorder randomness or uncertainty. Heat transfer from or to a heat reservoir.
The PH is around 9. Why does entropy increase. When we consider the nature entropy keeps on increasing.
A recent survey study mean age 155 yrs done by the HRC and the University of Connecticut shows a huge number of females who identify as trans boys We have to make the reasonable assumption at least a vast majority of trans boys are biological females. Entropy is defined as a measure of unusable energy within a closed or isolated system the universe for example. It turns out entropy is a pretty good way to explain times arrow.
With the increase in disorder that we think must have taken place being somehow. This part intrigues me and I feel this is the route I was trying to walk to discover why entropy is defined that way but still it starts as if dS is something that has always existed in physics or that is easily understandable. Temperature comes into play when the entropy and enthalpy both increase or both decrease.
Example 2 gives some indication of how an increase in entropy results in less heat transfer into work. Classical mechanics electromagnetism relativity quantum mechanics are time reversible. Lets say each solid has 3 atoms.
5 Calculation of Entropy Change in Some Basic Processes. We can now begin to answer the question we set out with. The entropy of vaporization is a state when there is an increase in entropy as liquid changes into a vapour.
As usable energy decreases and unusable energy increases entropy increases. Entropy will always increase on its own. This is because CH3COOH reacts with OH- produced water and CH3OO- which is a.
Something like mass which from a certain point of view doesnt need much explanation at least on a simpler level. We infer that a chemical reaction occurs spontaneously if it induces an increase in. The base 2 for the calculation of entropy.
Another important thermodynamic concept is that of entropy. Entropy is one of the few quantities in the physical sciences that require a particular direction for time sometimes called an arrow of timeAs one goes forward in time the second law of thermodynamics says the entropy of an isolated system can increase but not decrease. So clearly if you add heat to an ideal gas in an isothermal process entropy increase is not due to increased motion of the molecules.
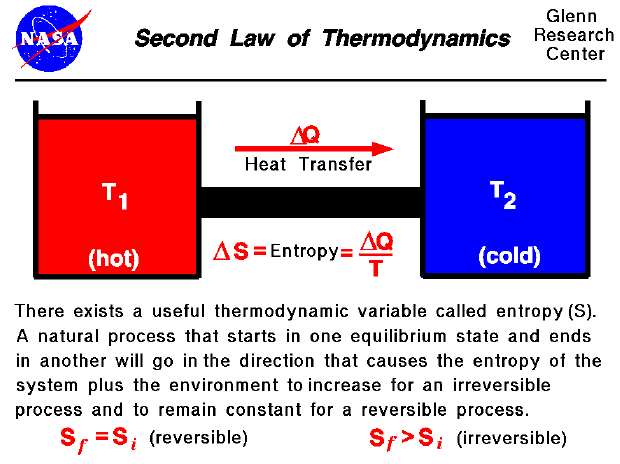
A heat reservoir Figure 53 is a constant temperature heat source or sinkBecause the temperature is uniform there is no heat transfer across a finite temperature difference and. Enthalpy is the sum total of all the energies whereas entropy is the measure of the change in enthalpytemperature. Adding entropy to requests.
Consider the human body. Theres something else going on. Entropy is all about arrangements of things but the actual things being arranged can be different in each case.
The Second Law of Thermodynamics states that the total entropy of a system and its surroundings always increases for a spontaneous process. This is due to an increase in molecular movement which creates a randomness of motion. Because entropy like energy is an extensive property a dilute solution of a given substance may well possess a smaller entropy than the same volume of a more concentrated solution but the entropy per mole of solute the molar entropy will of course always increase as the solution becomes more dilute.
One reason is that entropy is directly related to the fact that not all heat transfer can be converted into work. Entropy is also a gauge of randomness or chaos within a closed system. To see why were going to need two solids and stick them together.
Entropy is a measure of the energy dispersal in the system. Why Life is Remarkable. At first glance this law appears to contradict much common experience.
To predict whether or not a change will take place we need to take account of the entropy changes in the system and its surroundings. The study failed to verify biological sex which demonstrates the problems that arise when scientific research becomes affected. As usable energy is irretrievably lost disorganization randomness and chaos.
This principle explains for example why you cant unscramble an egg. Entropy in Daily Life. Entropy a measure of disorder explains why life seems to get more not less complicated as time goes on.
The Second Law of Thermodynamics says in simple terms entropy always increases. All things trend toward disorder. Therefore nature is spontaneous.
Recall that the second law of thermodynamics states that the total entropy or the entropy of the entire universe is constantly increasing. Entropy helps explain many of the mysteries and experiences of daily life. The entropy of vaporization is equal to the enthalpy of vaporization divided by boiling point.
Entropy always increases in irreversible processes. Once a resolver does enforce basic sanity checks an attacker has to flood the victim resolver with responses in an effort to match the query ID UDP port of the request IP address of the response and query name of. Note that while the entropy of the chemical system has decreased the total entropy entropy of system entropy of surroundings has increased ΔS total is positive.
The solid wood burns and becomes ash smoke and gases all of which spread energy outwards more easily than the solid fuel. 21 Example Suppose you have a random variable X such that. According to the equation when the entropy decreases and enthalpy increases the free energy change Delta G_ is positive and not spontaneous and it does not matter what the temperature of the system is.
Entropy is a measure of the level of randomness or disorder in a system. Relation Between Entropy And Enthalpy.
Principle Of Increase Of Entropy
Principle Of Increase Of Entropy

Entropy Button Zazzle Com In 2021 Entropy Thermodynamics Custom Buttons

How Could We Clearly Define Thermodynamics For Our Babies It S Not Always Easy To Teach Our Baby About The Thermodynamics Second Law Of Thermodynamics Entropy

Pin By Atanu Mondal On Chemistry Classroom Mind Map Thermodynamics Chemistry Classroom

Does Entropy Increase With A Decrease Or With An Increase In A System S Temperature Physics Stack Exchange

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

The Second Law Of Thermodynamics Biology For Majors I

Graphic Illustrating The Differences Between Classical And Quantum Thermodynamic Systems Arrow Of Time Second Law Of Thermodynamics Quantum

What Is Entropy And Why Is It Always Increasing

Entropy S It S Always Increasing Entropy Entropy Quote Arrow Of Time

Increase In Entropy Principle Updated 5 18 11

Application Area Of Thermodynamics Thermodynamics Chemistry Lessons Physics Lessons

Changes In The Overall Entropy Of A Single Biological Organism During Lifetime

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics